For investors only
All about the drug candidate arfolitixorin
and related information
Phase III AGENT study (Aborted)
Study design
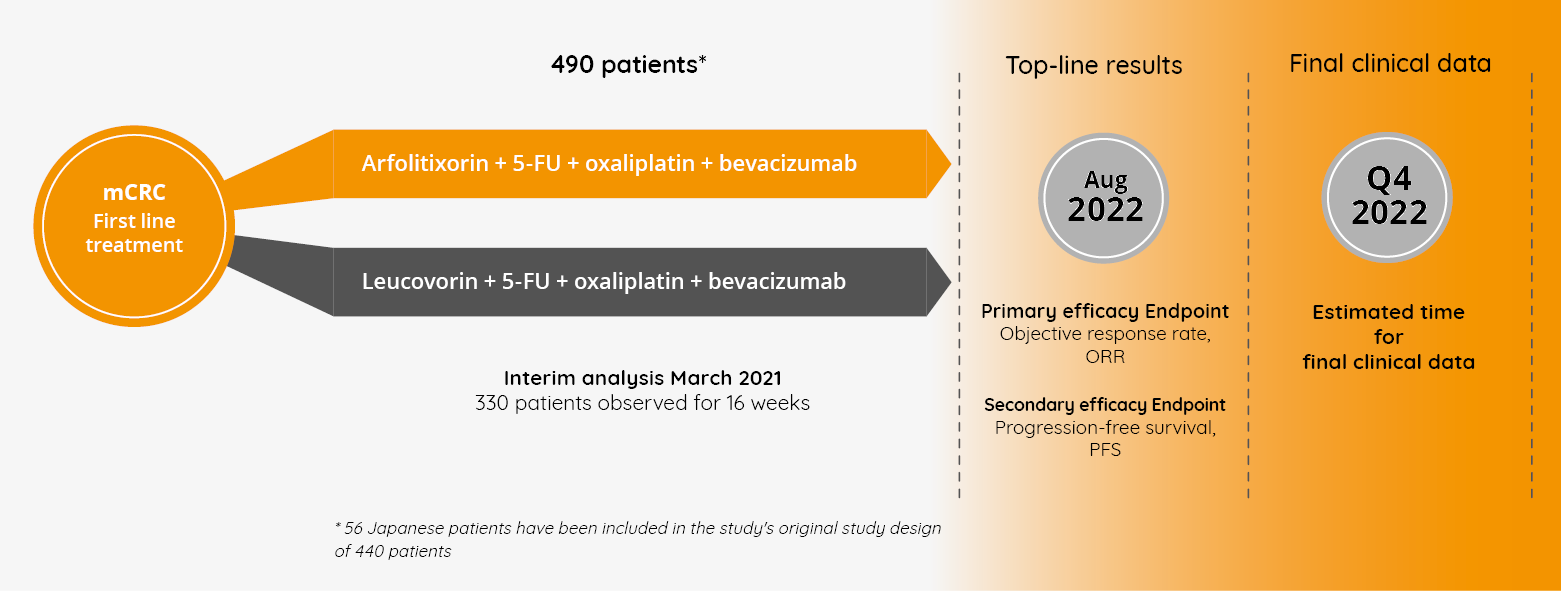
The AGENT study was a randomized, controlled, multi-center Phase III study assessing the efficacy and safety of arfolitixorin compared to leucovorin (the current folate-based treatment), both used in combination with 5-FU, oxaliplatin, and bevacizumab in first-line metastatic colorectal cancer (mCRC) patients. The study, which began in 2018, was the first study in around 20 years to investigate a meaningful alternative to the current standard treatment for the vast majority of mCRC patients and included approximately 90 clinics in the US, Canada, Europe, Australia and Japan and a total of nearly 500 patients. The primary endpoint of the study was to demonstrate that arfolitixorin was superior to leucovorin in terms of objective response rate (ORR). Both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) approved the study as the basis for an application for market registration (MAA, Marketing Authorization Application in Europe and NDA, New Drug Application in the US), provided that the study results demonstrate a statistically significant improvement in ORR compared to the control arm.
Quicker process through IND and Fast Track Designation
Isofol managed to significantly accelerate the study through approval from the pharmaceutical authorities (the FDA in the US and the EMA in Europe) to move directly from a Phase I/IIa study to the global Phase III AGENT study as an investigational new drug (IND) in the US. The company also later received a Fast Track Designation from the FDA based on the potential for arfolitixorin to address a large unmet medical need for new and more effective treatments of advanced CRC. The administration’s goal with this designation is to ensure that new treatments can become available faster for patients with serious diseases. For Isofol, it was also an important external validation of the potential and the need.
Negative study results
The top-line results from the study were presented at the beginning of August 2022. They indicated that arfolitixorin’s superiority to the control arm at the given dosage was not statistically significant. The company thus made the decision to not continue the study.
In-depth analysis of AGENT data
After the AGENT study was concluded, Isofol and external experts carried out a comprehensive analysis of the study data and came to the following conclusions:
• The chosen dosage regimen of two bolus doses likely meant that the concentration of arfolitixorin in the blood was too low to deliver a sufficiently high quantity
of the active ingredient into the tumor.
• The low dose meant that comparison with the control group with a standard treatment was not accurate, since the control group was being treated with a
higher dose.
• Pharmacokinetic models and a review of the available safety data indicate that it is likely possible to administer arfolitixorin at a higher dose than the one
evaluated in the AGENT study and that another dosage regimen would likely have boosted the effect of the drug candidate.
The conclusions from the analysis were that the dose of arfolitixorin was likely too low to allow for an accurate comparison with the control group and that the pharmaceutical ingredient was likely not optimally administered. The analysis did not indicate any sign that the safety profile for arfolitixorin, which was a prioritized part of the analysis, would prevent continued development of the drug candidate.
Last updated 05-20-2024
